Occurrence of Selective Ritonavir Nonadherence and Dose-Staggering
Results
Between June 2006 and February 2007, 45 subjects enrolled in the study. Thirty-six (80%) subjects completed the study. Of those who failed to complete, three lost or failed to return their caps, two decided not to use the caps at all, one began to use a pillbox shortly after enrollment, one never returned after the first visit, one switched to an efavirenz-based regimen, and one developed lymphoma early in the study and did not consistently use the MEMS caps because of frequent hospitalizations. Study completers contributed a mean of 18.9 weeks of adherence data. Common reasons for failing to provide a full 24 weeks of data included hospitalization, forgetting to replace the cap on new bottles of medication, traveling away from home without the MEMS monitor, and not completing the final study visit within the predefined acceptable 23-week window. There were no significant differences between study completers and noncompleters in terms of age, gender, HIV risk behavior, years living with HIV infection, prevalence of AIDS, cumulative time on HAART, or lowest documented CD4+lymphocyte count. There was a trend toward lower enrollment CD4+lymphocyte count in noncompleters as compared to completers (311 vs. 479 cells/mm, p=.07).
Demographic and clinical characteristics of the study sample are summarized in Table 1. All subjects were HAART-experienced at the time of study entry. Three subjects initiated their boosted atazanavir or fosamprenavir regimens on the day of enrollment. For the entire cohort, the mean length of time on boosted atazanavir or fosamprenavir prior to study enrollment was 460±390 days (range 0 to 1324).
Selective Nonadherence to Ritonavir
There was no significant difference in mean overall adherence to ritonavir (76.5% ± 27.4%) as compared to the boosted PIs (76.7% ± 27.4%). Three (8.3%; 95% CI 1.8%–22.5%) of the 36 subjects were selectively nonadherent to ritonavir. Two of them were receiving boosted fosamprenavir (one on a once-daily and one on a twice-daily schedule) and one was receiving boosted atazanavir. The mean difference in adherence between the boosted PI and ritonavir in these three subjects was 12.1% (range 5.5%–21.1%), and their mean adherence rate to the boosted PI was 93.3% (range 84.7%–99.4%). Of note, the subject with the highest rate of selective nonadherence to ritonavir was the one individual receiving her regimen on a twice-daily schedule. Thirteen subjects (36.1%; 95% CI 20.8–53.8) had perfect concordance of ritonavir and boosted PI adherence, and 31 (86.1%; 95% CI 70.5–95.3) had adherence differences between the two medications of less than 2%. One subject (2.8%; 95% CI 0.7–14.5), who was receiving boosted atazanavir, was selectively nonadherent to the boosted PI. The three subjects who were selectively nonadherent to ritonavir entered the study with VL <75 copies/mL, and all had VL <75 copies/mL at 24 weeks.
No subjects acknowledged selective nonadherence to ritonavir on self-report. Two subjects acknowledged selective nonadherence to both PIs together (and reported taking their other antiretroviral medications) on self-report.
Staggering of Ritonavir Doses
Seventeen (47.2%; 95% CI 30.4–64.5) subjects staggered any doses of ritonavir. In particular, eight subjects (22.2%; 95% CI 10.1–39.2) staggered any ritonavir doses before their boosted PI, five subjects (13.9%; 95% CI 4.7–29.5) staggered any ritonavir doses after their boosted PI, and four subjects (11.1%; 95% CI 3.1–26.1) staggered doses both before and after their boosted PI. For those staggering ritonavir doses before their boosted PI, the ritonavir was taken a mean of 6.6 ± 2.5 hours before the boosted PI; for those staggering ritonavir doses after their boosted PI, the ritonavir was taken a mean of 8.2 ± 2.5 hours after the boosted PI. In three (8.3%; 95% CI 1.8–22.5) of the 36 subjects, more than 5% of their boosted PI doses were accompanied by a staggered ritonavir dose. Two of these subjects were also selectively nonadherent to ritonavir. One of the three subjects took almost all staggered ritonavir doses after its associated boosted PI dose, and the other two subjects had approximately equal distributions of staggered doses before and after their boosted PI doses. There was no statistically significant correlation between percentage of doses staggered and VL at 24 weeks.
Surprisingly, 16 subjects, representing 44.4% (95% CI 27.9–61.9) of the entire cohort, appear to have taken more than 5% of their ritonavir doses more than 5 minutes apart from their associated boosted PI doses.
Relationship between Adherence to the Boosted PI and Virologic Outcomes
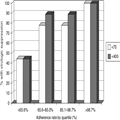
The mean adherence rate to the boosted PI for the study cohort was 76.7% ± 27.4%. Twelve subjects (33.3%; 95% CI 18.6–51.0) maintained adherence levels of greater than 95% over 24 weeks of monitoring, and three subjects were 100% adherent throughout that time. Twenty-seven (75%; 95% CI 57.8–87.9) subjects had VL <75 copies/mL after 24 weeks of adherence monitoring, and 29 (80.6%; 95% CI 64.0–91.8) had VL <400 copies/mL. There was a signifi cant inverse correlation in the relationship between adherence and VL at 24 weeks (Spearman's rho = −0.503, p=.002). The mean adherence of those with VL <75 copies/mL was significantly higher than those with VL ≥75 copies/mL (86.1 ± 15.7% vs. 48.7 ± 36.2%; p=.005), and was higher in those with VL <400 copies/mL compared to those with VL ≥400 copies/mL (85.9 ± 15.6% vs. 38.7 ± 34.1%; p=.002). The relationship of virologic outcome to adherence quartile is depicted in Figure 1.
(Enlarge Image)
Figure 1.
Rates of virologic suppression at 24 weeks according to adherence quartile.
